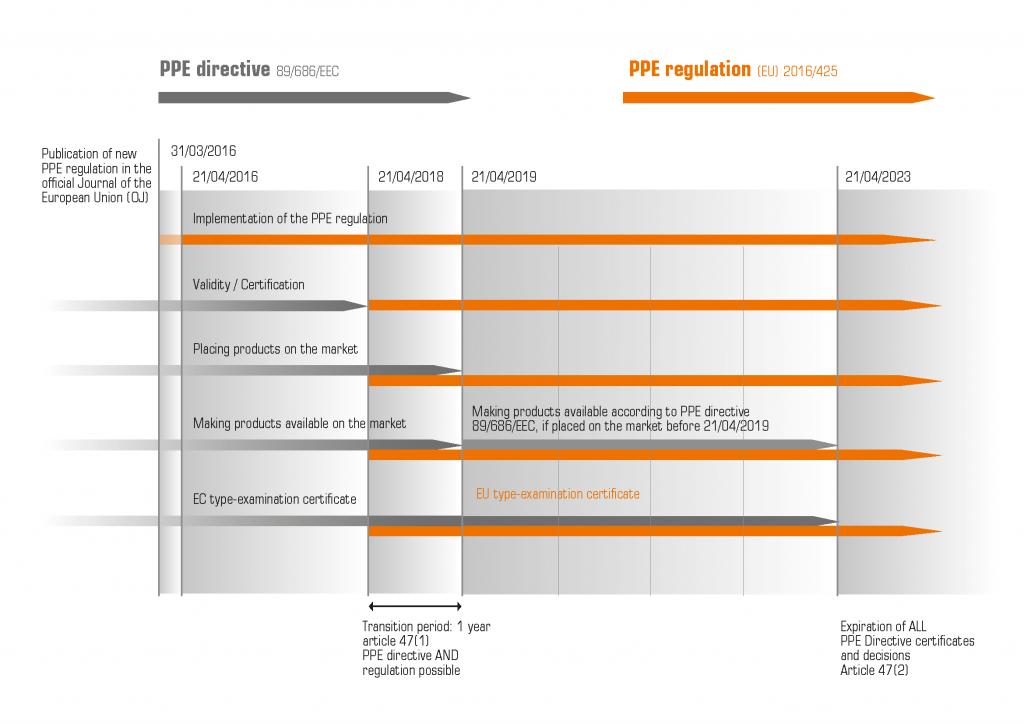
The new PPE Regulation (EU) 2016/425 replaced the 30-year-old PPE Directive 89/686/EEC of 1989 on 21 April 2018. It is binding in all EU Member States. On Sunday, 21 April 2019, the transitional period for manufacturers to place on the market Personal Protective Equipment (PPE) that does not meet the requirements of the Regulation, will end.
What are the specific consequences for market participants and what answers does SKYLOTEC have?
Declaration of Conformity: Each product must be accompanied by a Declaration of Conformity and the customer must be able to find it easily and quickly without a separate request to the manufacturer.
SKYLOTEC refers to the PPE Regulation in all instructions for use. The full Declaration of Conformity is available via this link.
Product labelling: Each product must bear the name and full address of the manufacturer.
SKYLOTEC has implemented this for all products where space is available, and specifies both the month and year of construction.
Obligations of importers and retailers: Compared to the existing Directive, the new Regulation imposes more obligations on all market participants. Thus there are also consequences for retailers, importers or other resellers. They, too, must ensure that the products they trade meet the current requirements and that the corresponding documentation is enclosed with the product.
When trading with SKYLOTEC, market participants can be sure that all CE-marked products that are accompanied by the prescribed documents comply with the regulations and can therefore be traded. If the offer (and thus the first "making available" of the product on the market) is made before the end of the transitional period, stock according to the PPE Directive can still be sold off according to the current legal interpretation of the European Commission.
Definition of the manufacturer: The PPE Regulation precisely defines the terms used by all economic operators. With immediate effect, the manufacturer who not only manufactures his products himself, but also has them manufactured and sells them under his name or brand is also considered to be the manufacturer. A product is deemed to have been placed on the market when an importer or retailer makes it available on the market in the EU for the first time. In this case, these market participants have the same obligations as a manufacturer in the strict sense.
SKYLOTEC is responsible for the role of the manufacturer according to the PPE Regulation and is therefore responsible for the conformity assessment procedure of all products placed on the market for the first time.
Validity of EU type examination certificates: The period of validity of EU type examination certificates is now limited to 5 years and must therefore be renewed regularly. EC type examination certificates were issued at the latest until 20 April 2018, which results in the maximum validity period until 21 April 2023.
SKYLOTEC has checked the type examination certificates of all products which have been certified according to the old directive until 21 April 2018. If these comply with the requirements of the new regulation, they will therefore retain their 5-year validity until 20 April 2023.
Use and sale of PPE according to the old directive: For all first-time distributors, which include EU importers or manufacturers such as SKYLOTEC, the transitional period during which products still certified according to the old PPE Directive may be sold, expires on 20 April 2019. According to the current legal interpretation of the European Commission, existing stock of products offered before the end of the transitional period (and thus the first "making available" of the product on the market took place before 20 April 2019) can still be sold off.
Retailers and resellers may still sell products in stock until 21 April 2023 if the protective function is still guaranteed and the expiry date is not exceeded. Users can, of course, also continue to use old products that comply with the directive for the entire usual or indicated service life.
Here you will find a link to the official guidelines of the PPE Regulation.
Summary at a glance:
Source: DEKRA, SAKRA
If you have any further questions regarding the PPE Regulation, please do not hesitate to contact us and we will be happy to help you!
E-Mail: [email protected]
Tel. : +49 (0)2631 · 9680-0

